About Sirio
Our production sites and companies worldwide
SIRIO Europe operates state-of-the-art facilities strategically located across Europe, Asia, and North America. With research and development centres, manufacturing sites, and product quality centres serving customers globally, SIRIO is a truly international nutraceutical CDMO supporting partners across key markets.
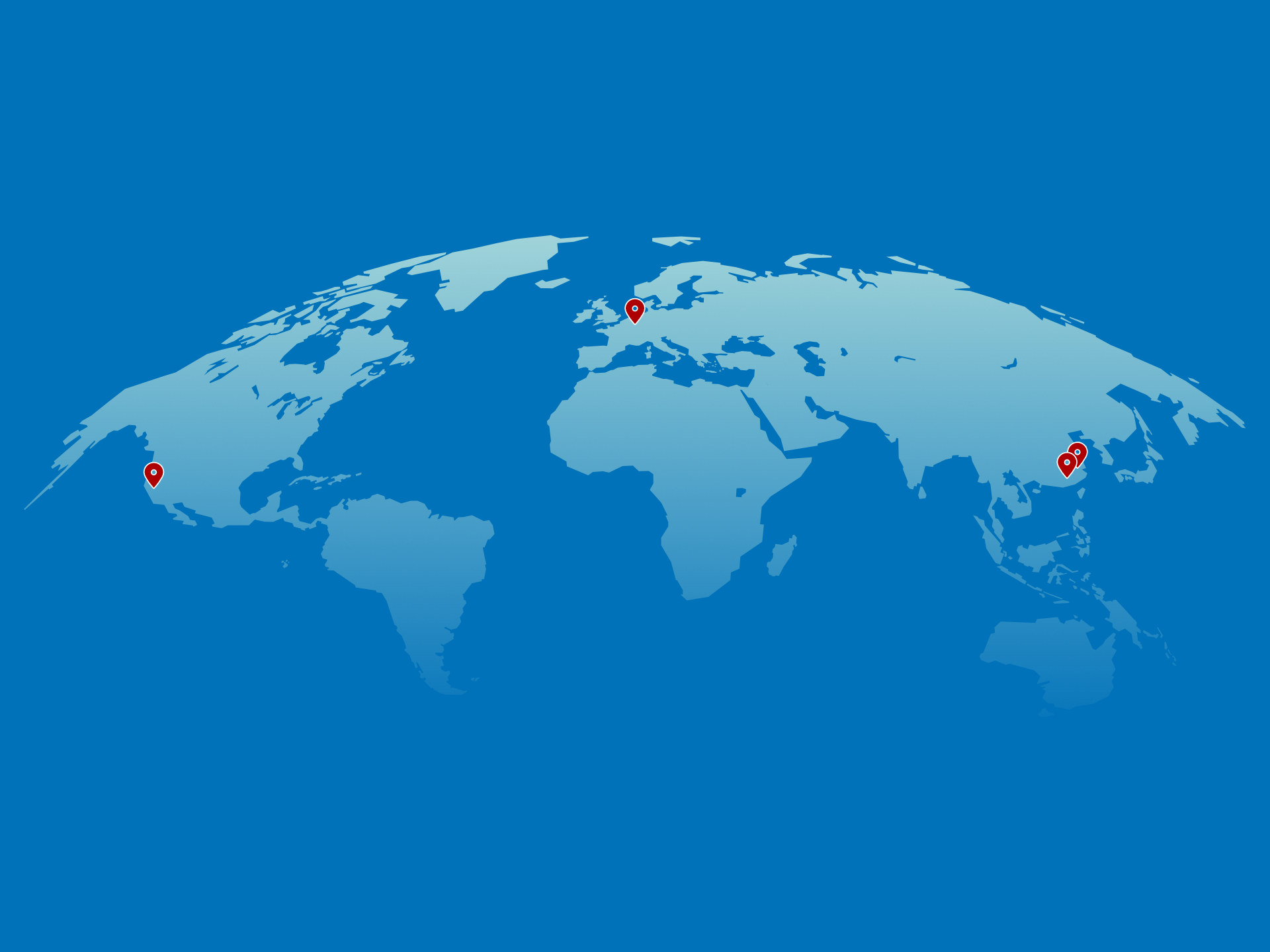
Global Nutraceutical CDMO
Our end-to-end capabilities — from formulation and development to manufacturing and packaging — are supported by a strong international footprint and robust quality systems, enabling reliable and scalable market access for our customers.
Headquarters
SIRIO Europe GmbH & Co KG
Messeturm, Friedrich-Ebert-Anlage 49
Floor 24, 60308 Frankfurt am Main

European Headquarters & Commercial Office
SIRIO Europe’s European headquarters is located in Frankfurt, Germany, and serves as the group’s central commercial office for the European region. The Frankfurt office coordinates sales, marketing, business development, and customer engagement activities across Europe, working closely with SIRIO’s manufacturing and R&D sites within the global network.
As the commercial hub for Europe, the Frankfurt headquarters supports customers throughout the region by providing market access, project coordination, and interface with technical, regulatory, and production teams. The office plays a key role in aligning regional customer requirements with SIRIO’s global capabilities while ensuring compliance with applicable regulatory and quality standards.
Manufacturing facility
SIRIO Europe GmbH & Co KG
Am Hünengrab 20
16928 Pritzwalk, Germany
Phone: +49 33986 636 0
Fax: +49 33986 636 999

Global Market, Local Supply
Founded in 1992 and headquartered in Pritzwalk, Germany, SIRIO’s European headquarters integrates commercial and manufacturing activities. The site serves around 150 customers in approximately 50 countries and is well recognised within the European dietary supplement industry.
The German manufacturing site, Sirio Pharma Germany GmbH, is GMP-approved and focuses on the development and production of complex softgel dosage forms. Separate encapsulation lines for nutraceutical and pharmaceutical softgels are available.
Sirio Pharma Germany GmbH
Dosage forms
-
Pharmaceutical softgels
-
Nutraceutical softgels
-
Probiotic softgels
-
Vegetarian softgels
R&D & Quality
-
Softgel technology platforms and formulation development
-
Quality Control (QC) laboratories
-
In-process control (IPC) and final product testing
-
Raw material testing and release
-
Quality Assurance (QA)
-
Fully equipped analytical laboratories
-
GMP and other applicable quality certifications
SIRIO Pharma Co. LTD
SiRIO Healthcare (Anhui) Co.LTD
Manufacturing, R&D
No. 1980 South Hongqi Road Ma’anShan Economic and Technological Development Area, Anhui Province, China.

Ma’anshan Manufacturing Facility (China)
The Ma’anshan site is a smart manufacturing facility integrating R&D, testing, production, and intelligent warehousing. It provides end-to-end capabilities from product development to large-scale manufacturing and packaging, supporting a wide range of nutraceutical dosage forms.
In 2023, the facility was recognized as both a “Smart Factory” and a “Green Factory” in Anhui Province.
Key capabilities include:
-
Large-scale customized production alongside flexible small-batch manufacturing
-
Fully digitalized logistics and warehouse management to improve operational efficiency
Production scope:
-
Gummies
-
Liquids and beverages
-
Powders
-
Tablets and capsules
-
Probiotic dosage forms
SIRIO Pharma Co. LTD
SIRIO Pharma Co. LTD
No. 83 Taishan Rd.,
Shantou Guangdong, China.
SiRIO Healthcare (Anhui) Co.LTD
Manufacturing, R&D
No. 1980 South Hongqi Road Ma’anShan Economic and Technological Development Area, Anhui Province, China.

Two manufacturing plants are based in Shantou, China.
Shantou is home to two manufacturing plants and a dedicated research and development centre. These facilities support large-scale production and advanced quality management, incorporating smart manufacturing technologies and digitalised processes.
Manufacturing capabilities
-
Softgels
-
Vegetarian softgels
-
Powders
-
Hard capsules
-
Tablets (including effervescent and chewable formats)
-
Probiotic products across multiple dosage forms
-
Packaging solutions including bags, bottles, and blisters
Product & quality focus
-
Gummies
-
Functional beverages
-
Powders
-
Probiotic dosage forms
-
Lightweight packaging solutions
R&D Centre
-
Purpose-built R&D facility designed to international standards
-
Dedicated pilot production lines for softgels, powders, and tablets
-
Analytical Centre (Quality)
-
Quality Control (QC) and Quality Assurance (QA) laboratories
-
Broad range of advanced analytical equipment
-
Extensive portfolio of validated testing methods
-
Compliance with applicable quality and certification standards
SIRIO China
Shanghai Office
Room 3303, 33F, T1, Raffles City,
No.1133, Changning Road Shanghai, China

SIRIO Americas
SIRIO USA Headquarters
18300 Von Karman Avenue I Suite 750
Irvine, CA 92612, California, USA.

SIRIO’s U.S. facilities support product development through formulation services, analytical testing, and regulatory assistance. The sites are designed to support pilot production, scale-up activities, and in-house testing in line with applicable quality and regulatory standards.
Key capabilities include:
-
Formulation & Product Development
-
Non-GMO and gluten-free formulations
-
Plant-based, vegetarian, and vegan solutions
-
Excipient-free and clean-label development
-
-
Pilot & Scale-Up Services
-
Support for pilot production and scale-up activities
-
-
Analytical & Quality Capabilities
-
Physical, chemical, and microbiological testing
-
Analytical method development and validation
-
Accelerated stability and shelf-life evaluation
-
Zhuhain, China
Zhuhai Bonded Factory
2F&4F, Zone A, Yinji Cross-border Complex Building, 37# Warehouse, Zhongxin Rd.
Zhuhai Free Trade Zone, Xiangzhou District, Zhuhai, Guangdong Province, China

Cross-border business support (Zhuhai–Macau)
Located in the Zhuhai–Macau Cross-Border Industrial Area, this facility provides integrated cross-border business support within SIRIO’s global production network. Key operational functions include procurement, logistics, R&D coordination, manufacturing support, packaging, and regulatory compliance.
-
Utilisation of cross-border policy advantages to support efficient logistics
-
Advanced automated production and packaging lines
-
Integrated quality testing and certification support, including COA documentation and CNAS reports
-
Production and packaging of softgels, hard capsules, tablets, and personalised nutrition packs