About Sirio
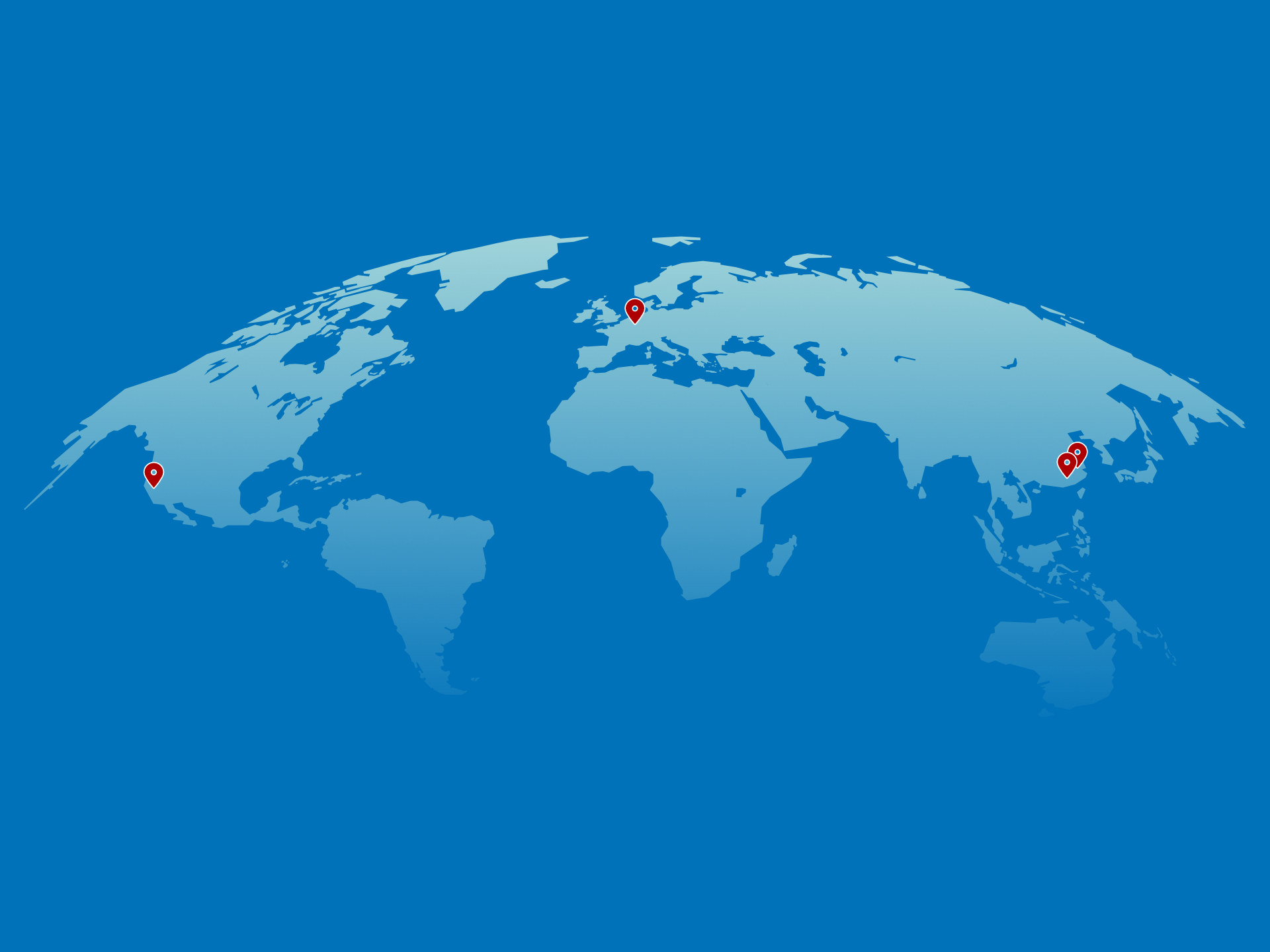
Our production sites and companies worldwide
SIRIO Europe has state-of-the-art facilities strategically located across Europe, Asia and North America. With research and development centres, manufacturing plants and product quality centres serving customers across the world, SIRIO is a truly global organisation, perfectly position to serve the global nutraceutical market.
Global Nutraceutical Leading CDMO
Our end-to-end services, from formulation to packaging, and our global footprint mean you can get to market faster while our quality standards and certifications ensure your peace of mind.
Europe HQ
SIRIO Europe GmbH & Co KG
Am Hünengrab 20
16928 Pritzwalk, Germany
Commercial office in Frankfurt
Phone: +49 33986 636 0
Fax: +49 33986 636 999

Global Market, Local Supply
Founded in 1992 and headquartered in Pritzwalk, Germany, the European HQ has commercial and manufacturing activities, serving around 150 customers in 50 countries. It is well recognised in the European dietary supplement industry. The German manufacturing site, Sirio Pharma Germany GmbH, is pharma GMP approved. Siro Pharma Germany focuses on development and production of complex softgels. Separate encapsulation lines for nutraceutical and pharmaceutical softgels are available.
Sirio Pharma Germany GmbH
- Pharmaceutical softgels
- Nutraceutical softgels
- Probiotic softgels
- Vegetarian softgels
R&D & Quality
- Softgel technology platforms & formulation
- Quality control (QC) laboratories
- IPC and final product testing
- Raw material testing and release
- Quality Assurance (QA)
- Fully equipped laboratory for softgel analytics
- GMP & other quality certifications
SIRIO Pharma Co. LTD
SIRIO Pharma Co. LTD
No. 83 Taishan Rd.,
Shantou Guangdong, China.
SiRIO Healthcare (Anhui) Co.LTD
Manufacturing, R&D
No. 1980 South Hongqi Road Ma’anShan Economic and Technological Development Area, Anhui Province, China.

- Phone: +86 (754) 8892 4286 (Domestic business)
- +86 (754) 8998 3900 (Overseas business)
- Fax:+86-754-8881 0328
- sales@siriopharma.com (for domestic matter)
- intl@siriopharma.com (for international matter)
- sourcing@siriopharma.com (for suppliers)
Two manufacturing plants are based in Shantou, China.
In addition to being SIRIO’s global head office, Shantou is also home to two manufacturing plants that produce softgels, tablets, packaging and more and a state-of-the-art research and development centre.
Industrial Internet of Things (IIoT) and smart manufacturing: We use digital and other innovative technologies to run our operation and production and depend less on human intervention.
Shantou Manufacturing Plants
- Softgels
- Vegetarian softgels
- Powder
- Hard capsules
- Tablets (incl. efferescent, chewable)
- Probiotic products in various dosage forms (gummy, capsules, chewable tablets, powder)
- Packaging (bags, bottles, blisters)
- Factory area: 240,000 m²
Production and Product Quality Center for:
- Gummies
- Functional beverages
- Powder
- Probiotic dosage forms
- Packaging solutions: Lightweight bags and bottles
- Probiotic products in various dosage forms (gummy, capsules, chewable tablets, powder)
- Packaging (bags, bottles, blisters)
R&D Centre
- 2,600 m², designed by German architects
- Equipped with pilot production lines for softgels, powder and tablets
- Analytical Centre (Quality)
- Quality control (QC) and quality assurance (QA)
- State of the art equipment: HPLC, gas chromatography, atomic absorption spectrometer, infrared spectrometer, amino acid analyzer and much more.
- 2000 test methods
- Quality & Certifications
- Quality control & Quality Assurance
- Fully equipped laboratories
- Quality & Certifications
SIRIO Shanghai
Shanghai Office
Room 3303, 33F, T1, Raffles City,
No.1133, Changning Road Shanghai, China
R&D Center in planning

Sirio Americas
SIRIO USA HQ
18300 Von Karman Avenue I Suite 750
Irvine, CA 92612, California, USA.